


split the above string into an array of strings Let us take a look at this using an example const str = 'i have learned something new today' To achieve this, we split the words from the string and store them in an array, and then use the above concept on each element of the array and join all the array elements together to get the string back. Capitalize the first letter of each word in a string For example, the ionic compound, lithium chloride. Now what if we want to capitalize the first letters of all the words in a string? Let’s see how we can achieve this as well. Like the partial ionic character in covalent compounds, ionic compounds show partial covalent character. const str = 'flexiple' Ĭonst str2 = str.charAt(0).toUpperCase() + str.slice(1) Īs you can see from the above outputs that we have capitalized the first letter of the input string. Now let us use all the three functions together to capitalize the first word of an input string. This function slices a given string from a specified “start” position until the specified “end” position. Now, we would get the remaining characters of the string using the slice() function. To capitalize the first character of a string, We can use the charAt() to separate the first character and then use the toUpperCase() function to capitalize it. In the above example, we see that using the toUpperCase() function on the string converted all the characters of the input string to capital letters.īut this is not what we desire to achieve. The toUpperCase() function converts all the characters of an input string to uppercase The charAt() function returns the character at a given position in a string. To achieve the capitalization of the first letter of a given string in JavaScript, we would use three functions. Capitalize the first letter of each word in a string 60K views Ionic Character and Electronegativity To determine when bonds are ionic, polar covalent, or non-polar covalent (these options are known as ionic character ), it is essential to have.Capitalize the first letter of a string.
The ionic character of the alkali metal hydrides increases from Li to Cs. Consequently, the reactivity of the alkali towards hydrogen decreases down the group. On the other hand, C-Br and C-I are sometimes referred to as ionic (at least informally). Source 1 Explanation: As the size of the metal cation increases down the group, the lattice energy of the hydrides decreases down the group.

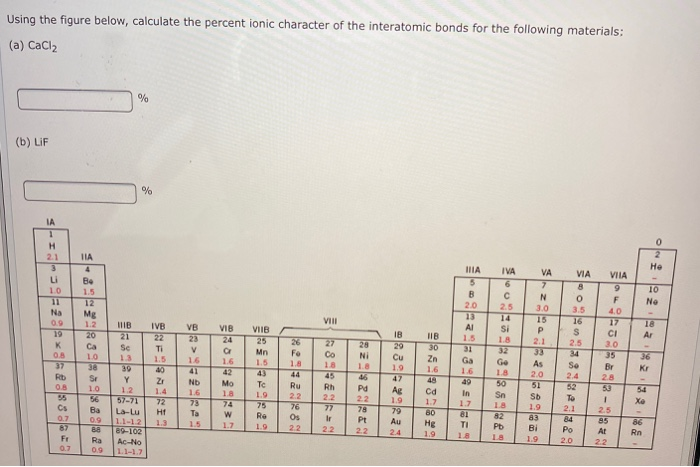
Electron-rich (negatively charged) regions are shown in blue electron-poor (positively charged) regions are shown in red. 1 Can ionic character also be construed as a poor sharing of electrons rather than simply as the taking of electrons For example, NaCl is ionic, and that's because the chlorine takes sodium's one valence electron. A polar covalent bond (b) is intermediate between the two extremes: the bonding electrons are shared unequally between the two atoms, and the electron distribution is asymmetrical with the electron density being greater around the more electronegative atom. In a purely ionic bond (c), an electron has been transferred completely from one atom to the other. In a purely covalent bond (a), the bonding electrons are shared equally between the atoms. Recall that a lowercase Greek delta ( δ) is used to indicate that a bonded atom possesses a partial positive charge, indicated by δ +, or a partial negative charge, indicated by δ −, and a bond between two atoms that possess partial charges is a polar bond.įigure 9.6.4 : The Electron Distribution in a Nonpolar Covalent Bond, a Polar Covalent Bond, and an Ionic Bond Using Lewis Electron Structures. Figure 9.6.4 compares the electron distribution in a polar covalent bond with those in an ideally covalent and an ideally ionic bond. Most compounds, however, have polar covalent bonds, which means that electrons are shared unequally between the bonded atoms. The two idealized extremes of chemical bonding: (1) ionic bonding-in which one or more electrons are transferred completely from one atom to another, and the resulting ions are held together by purely electrostatic forces-and (2) covalent bonding, in which electrons are shared equally between two atoms. Percent Ionic Character of a Covalent polar bond


 0 kommentar(er)
0 kommentar(er)
